ACID RAIN: A DEATH CALL.
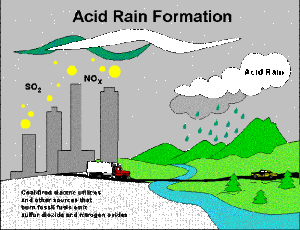
Acid rain refers to rain, or any form of precipitation that is acidic in nature, which means it contains hazardous compounds and causes a low pH leve (contains elevated levels of hydrogen ions). Acid rain has harmful effects on plants, animals and infrastructure. Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acid.
What causes this acidification of rain?
The most important compound resulting in the formation of acid rain is sulphur dioxide. Nitrogen oxides present in the atmosphere are oxidised to form nitric acid when then interferes with water molecules to result in acidic rain. The nitrous oxides are found in the emissions of combustion of fossil fuels and volcanic eruptions.
Acidic rain can occur in two ways:
v Natural Phenomena: By natural phenomena, we refer to the fact that man cannot do anything to stop it from happening and neither does he have any direct reason to its happening either. The natural phenomenon of acidic rain occurs due to volcanic eruptions and lightening. To further explain, in the process of lightening, electrical activity that is produces causes nitrogen to enter into the lower surface of the earth, when it rains this nitrous oxide combines with the water molecule to form nitric acid and thus the formation of acid rain.
v Human Activity: As in every other way man has to be responsible in some way or the other in destroying mother earth and to add to it he also contributes to the formation of acid rain which can also prove to be lethal. So how do we manage to do it? The emission of nitrogen and sulphur containing compounds are the main reason contributing to acid rain. Electricity generation, fumes from factories and industrial units and emissions from vehicles are only some of the few examples. The gases can be carried hundreds of kilometres in the atmosphere before they are converted to acids and deposited. In the past, factories had short funnels to let out smoke but this caused many problems locally; thus, factories now have taller smoke funnels. However, dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage.
The formation of acidic rain is a chemical process. Let me illustrate it with the help of chemical equations and the simple chemistry learnt in 10th grade:
Combustion of fuels produces sulphur dioxide and nitric oxides. They are converted into sulphuric acid and nitric acid.
Gas phase chemistry
In the gas phase sulphur dioxide is oxidized by reaction with the hydroxyl radical via an intermolecular reaction:
SO2 + OH· → HOSO2·
This is followed by:
HOSO2· + O2 → HO2· + SO3
In the presence of water, sulphur trioxide (SO3) is converted rapidly to sulphuric acid:
SO3 (g) + H2O (l) → H2SO4 (aq)
Nitrogen dioxide reacts with OH to form nitric acid:
NO2 + OH· → HNO3
Chemistry in cloud droplets
When clouds are present, the loss rate of SO2 is faster than can be explained by gas phase chemistry alone. This is due to reactions in the liquid water droplets.
Hydrolysis
Sulphur dioxide dissolves in water and then, like carbon dioxide, hydrolyses in a series of equilibrium reactions:
SO2 (g) + H2O is in equilibrium with SO2·H2O
SO2·H2O is in equilibrium with H+ + HSO3−
HSO3− is in equilibrium with H+ + SO32−
Oxidation
There are a large number of aqueous reactions that oxidize sulphur from S(IV) to S(VI), leading to the formation of sulphuric acid. The most important oxidation reactions are with ozone, hydrogen peroxide and oxygen (reactions with oxygen are catalysed by iron and manganese in the cloud droplets).
So above is the chemical procedure of the formation of acid rain. And how normal compounds when come in contact with water turn into acid and come down in the form of rain causing endless harm to the environment.
ACID DEPOSITION
This is an extremely important point and focus of this write up. We all believe that acid deposition is generally in the form of rain, which is not always true. Acid deposition may also be dry in nature. Let us look at the two briefly:
o Wet Deposition: This is the deposition of acid by precipitation in the form of rain, snow, etc., which we have extensively explained above. Wet removal of both gases and aerosols are both of importance for wet deposition.
o Dry Deposition: Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition. This occurs when particles and gases stick to the ground, plants or other surfaces.
Adverse effects
The term acid highlights that none of the above mentioned words are to be taken lightly. Acid on a man’s skin is so dangerous and even fatal, so just imagine if it were to rain acid on the surface of the earth, the effect would be disastrous.
- Surface water and aquatic animals: As lakes and rivers become more acidic biodiversity is reduced. Reduction in pH levels (below 5) do not cause fish eggs to hatch and extensive low levels of pH in water cause the death of various aquatic animals especially fishes.
- Soils: the fall of acidic rain causes a huge decline in pH levels and this causes a change for the microbes which find it difficult to survive in low pH levels.
- Forest and other vegetation: plants can also be damaged by acid rain, but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death.
- Human health effects: As contributor to acid rain we also are harmed by it. It does not directly affect us but it does enter the underground water cycle and eventually into our systems. They result in heart and lung problems mainly asthama and bronchitis.
This is our earth and we must do all that we can to protect it. In this universe we are probably the most advanced species. We must protect and guard our very source of existence. Reduce vehicular pollution. Do not emit highly hazardous industrial wastes into the air. Try and follow green living for a better future.

Leave a Reply